C02: FROM WASTE TO RESOURCE USING RENEWABLE ENERGY
The promise of renewable energy in chemicals production
In the coming 20 years, large changes will happen to make our society sustainable. Renewable energy is pivotal for this change. In the transition period, our industrial infrastructure should be able to cope with this shift from fossil to renewable. This can lead to issues such as power grid instabilities, but also opportunities arise like the ability to use renewable energy within the chemical industry.

 WHAT IS VOLTACHEM?
WHAT IS VOLTACHEM?
VoltaChem is a business-driven Shared Innovation Program that connects the electricity sector, equipment sector and the chemical industry. Together, new technologies and business models are developed and implemented that focus on the use of renewable energy in the production of heat, hydrogen and chemicals. Through our community and research activities, we serve and work with the industry to strengthen its competitive position and that of its suppliers.
More information: www.voltachem.com
The chemical industry is ideally suited to use electrical energy for their primary and secondary processes and is therefore one of the sectors that can also contribute to the Netherlands government goals as stated in the “Energy Agreement for Sustainable Growth". One of the objectives of this agreement is to increase the proportion of energy generated from renewable sources from 4.4% (2013) to 14% in 2020 and to 16% in 2023. A large portion of this renewable energy can be used for production purposes, especially in the chemical industry which constitutes the largest energy consumer. With more solar and wind energy being produced in North West Europe, new opportunities for the chemical industry arise. In this way, our commodity chemicals can be produced with a lower carbon footprint. From this context, the concept of Power-to-Chemicals is born.
POWER-TO-CHEMICALS: EFFICIENT CHEMICAL CONVERSIONS USING ELECTROCHEMISTRY
Within the inorganic chemistry sector, the industrial use of electricity is an established technology. For instance, the production of chlorine from salt or the production process for aluminum are direct electrochemical processes. However, in other parts of the chemical industry the use of electricity for conversion of feedstock, although scientifically possible, is still very limited. Why is this? It is of importance to note that for the (economic and ecological) assessment and comparison with traditional chemical routes many factors are important:
- Costs (operational and capital) from a system perspective.
- Feedstock and waste footprint.
- CO2 footprint.
In general, an electrochemical process can lead to higher selectivity and therefore to a higher product purity than a classical pathway. It can be stated that, as a rule of thumb, when existing processes are done with high efficiency and when the competing chemical pathway is based on catalytic processes using molecular oxygen and hydrogen, it will be likely that the electrochemical routes are not economically attractive. Bio based feedstocks can therefore be of particular interest as starting material in an electrochemical process.
ELECTROCHEMICAL CO2 CONVERSION: PROMISING PRODUCTS
Especially in the last decade, significant research has been done on the utilization of CO2 as a feedstock. Large scale transformation of CO2 to value added chemicals is of high interest from an economic and an environmental perspective. In addition to biomass it is the only way to introduce non-fossil carbon into the chemicals and fuels arena.

It has been shown that based on electrochemistry, a large variety of products can be made, see figure 1. It is of importance to note that these products are obtained from electrochemical conversion without taking process intensification into account. For some of the molecules, an indirect route is available (e.g. from the thermo-catalytic conversion of electrochemical hydrogen with CO2), for others, only a direct electrochemical route is available. Based on first principles, number of electrons needed and assessment of cell potential, it is possible to calculate the electricity cost which is a major part of operational expenditure. Figure 1 shows the assessment of direct electrolysis costs for different products obtained from CO2. Based on this assessment it can be concluded that it is economically not attractive to go for methane, that methanol is best produced through the electrochemical hydrogen route and that a positive business case for ethylene is very challenging due to the amount of electrons, and thus energy, needed. However, formic acid, CO and oxalic acid are from an economic perspective really promising products to be made directly through electrochemistry using available science and technology.
RECENT DEVELOPMENTS: PAIRED ELECTROSYNTHESIS AND CO2 CAPTURE-CONVERSION
In the last years, we have shown that with the concept of paired electrosynthesis, meaning simultaneous production of desired products at the cathode and anode, significant improvements can be achieved related to operational expenditures (OPEX) and capital investments (CAPEX). For example, in 2018 we have proven that electrochemical CO2 reduction to CO can be paired with the conversion of propylene glycol to lactic acid, drastically increasing the business case for both chemicals. Another notable example is the pairing of the CO2 reduction reaction with the well-established electrochemical chlorine production reaction. In paired electrosynthesis, with the same electron consumption and with the same equipment two specific and valuable products can be produced instead of one. Furthermore, the technology could be retrofitted in existing chlorine plants. Within the VoltaChem program a new project has been started recently with TNO and Avantium, in which this concept will be further developed together.


electrochemical conversion as proposed by the TNO researchers in the VoltaChem program
Another concept that can add economic value to the electrochemical conversion of CO2 is the integration of CO2 capture and electrochemical conversion. TNO researchers within the VoltaChem program are actively pursuing the direct use of CO2 capture solvents in the electrochemical reactor. The main gain of this approach is that it will lead to a process intensification where a separate CO2 stripping unit, which adds to equipment costs and which consumes a lot of energy, is not needed anymore.
CONCLUSION: REDEFINING THE PARADIGM OF CO2
In our CO2 electrochemical conversion research and development line Power-2-Chemicals we combine the production of renewable energy, electrochemical synthesis of valuable chemicals from CO2 and finally the capture of CO2. Individually, these technical building blocks are not competitive with current industrial practice yet. But worldwide technical and scientific developments of better performing technologies are accelerating rapidly. Furthermore, the business case of these different technologies lies not so much in their individual performance, but in their systemic integration into coherent processes. By combining technological building blocks in a smart way, viable business cases can already be created on short term, truly moving ahead and changing the paradigm of CO2 being a waste. Instead, in the near future, CO2 will be utilized as a precious resource. And that is exactly what we, together with our partners and members, strive for in the business-driven innovation VoltaChem program.
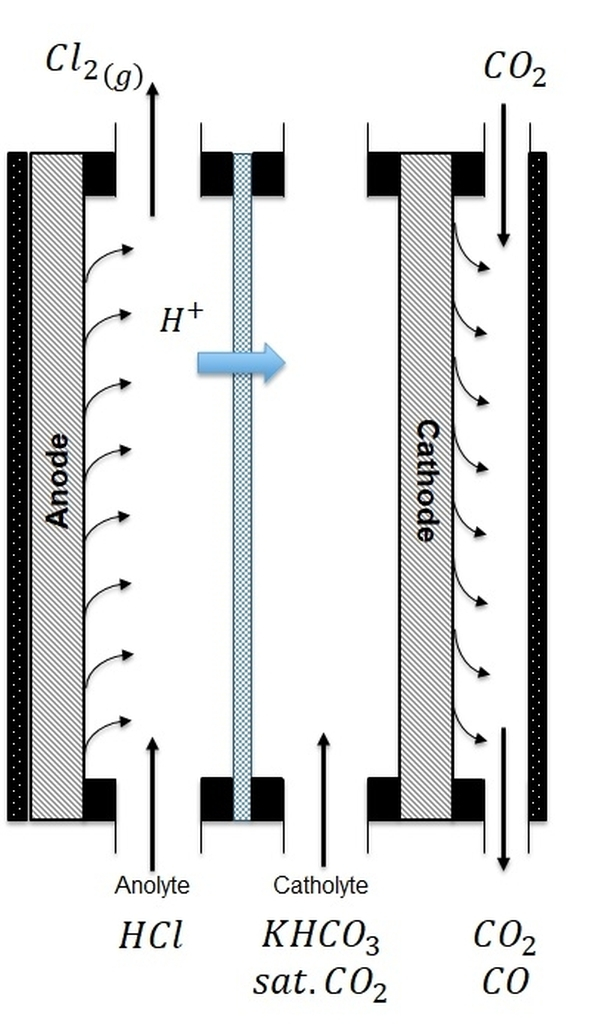 PAIRED ELECTROSYNTHESIS
PAIRED ELECTROSYNTHESIS
In an electrochemical reaction, the oxidation and the reduction processes occur simultaneously and are typically separated into different compartments of the reactor.
Typically, only one of the reaction products is of interest and the reciprocal reaction leads to a product that is not valorized (e.g. H2, O2).
In a paired electrolysis concept, both products obtained are meant to be valorized. The image on the left illustrates the paired production and therefore simultaneous production of CO (from CO2) and Cl2.
In the framework of the VoltaChem program, TNO researchers and industrial partners are developing paired electrolysis concepts such as the one illustrated here.
 Earl Goetheer is principal scientist process technology at TNO, part-time professor electrochemical CO2 transformation at TU Delft and scientifically responsible for the Power-2-Chemicals research line within VoltaChem. He was the originator of the direct carbon capture-conversion idea by combining his large experience in carbon capture and utilization with recent developments in power-to-X using electrochemistry.
Earl Goetheer is principal scientist process technology at TNO, part-time professor electrochemical CO2 transformation at TU Delft and scientifically responsible for the Power-2-Chemicals research line within VoltaChem. He was the originator of the direct carbon capture-conversion idea by combining his large experience in carbon capture and utilization with recent developments in power-to-X using electrochemistry.
 Anca Anastasopol is research scientist in TNO and technically responsible for the electrochemical CO2 conversion line within VoltaChem Power-2-Chemicals. In her role as system integrator she combines developments in the electrochemistry domain with electrochemical reactor engineering and scale-up.
Anca Anastasopol is research scientist in TNO and technically responsible for the electrochemical CO2 conversion line within VoltaChem Power-2-Chemicals. In her role as system integrator she combines developments in the electrochemistry domain with electrochemical reactor engineering and scale-up.

